Baking soda and vinegar fireworks experiment for kids.
Fireworks can be so much fun. They are festive and bright and just plain cool.
But they can also be loud, they are only around at certain times of the year, and there is that whole danger aspect of them.
But we can have some firework fun at home, without all the noise and danger. Just a simple chemical reaction from supplies you have in your kitchen. These DIY fireworks still wow preschoolers and toddlers, and they are safe and fun.
So let’s learn how to make baking soda and vinegar fireworks at home.
What's In This Post?
Baking Soda and Vinegar Firework Experiment
This is a great experiment to surprise your kids with. By that, I mean set up the first time without their help so they don’t know the glitter is going to appear. It adds to the wow factor like real fireworks.
I also particularly like this little experiment because it is much safer than actual fireworks. Yes, it is super cool to see them explode in sparks and colors, but I do not want that in my house. (Glitter can be a tricky craft supply since it tends to end up everywhere. So I suppose we hold on to a little bit of the danger by using that. 😉 )
Supplies for Your At Home Fireworks Experiment
You only need a few things to do this experiment, it’s so simple to pull together. Here’s what to get out:
- Baking Soda
- Vinegar
- Glitter
- Star Cookie Cutter (or another shape of choice)
- Dish To Hold Reaction
- Plastic Dropper
That’s it! Pretty simple.
How To Do the Fireworks Experiment with Baking Soda and Vinegar
This activity is fast to put together. Like I said before, I recommend setting this up for your kids the first time so they are surprised. After that let your child help set it up because they will want to do this over and over.
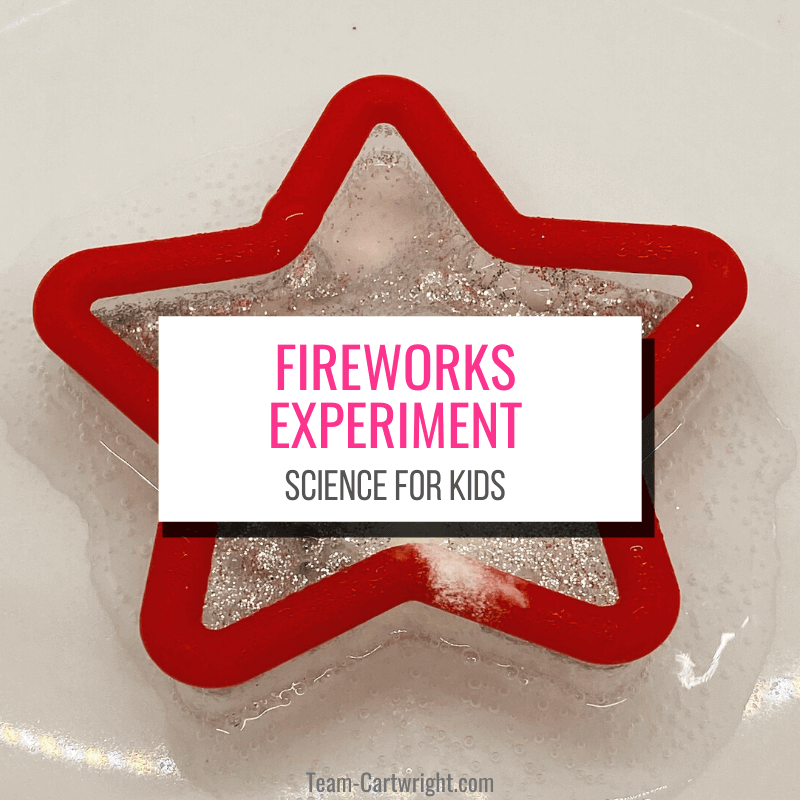
Step 1: Take your star cookie cutter and place it in a shallow dish.
Step 2: Sprinkle glitter in on the dish inside the cookie cutter.
Step 3: Add baking soda on top of the glitter so you cannot see any glitter.
Step 4: Give your child vinegar and a plastic dropper and have them add vinegar to the cookie cutter. The chemical reaction will reveal the glitter like a firework!
Clean Up and Tips:
Clean up for this is pretty easy. Simply wipe off the glitter from your dish and rinse any remaining baking soda and vinegar down the drain. Try not to get glitter down your drain.
Make sure you do this activity in a dish with sides. The vinegar can leak out under the cookie cutter and having it in a container will help stop messes. (Learn more about mess-free play here: Mess-Free Sensory Play Tips.)
You can use any shape cookie cutters you have for this. If you don’t have cookie cutters available you can also use muffin tins!
Once your firework reaction is complete you can wipe down the dish and try it again!
Try these fun activities too!
Safety
This is a pretty safe activity. Make sure your child doesn’t inhale the baking soda or the glitter and be sure they don’t eat any of the components. (The baking soda and vinegar are taste safe, but this is a good opportunity to practice safe lab techniques.)
The Science of the Fireworks
The science of this activity is pretty simple. It’s our favorite kitchen chemical reaction, baking soda and vinegar.
Baking Soda and Vinegar Reaction
The baking soda and vinegar reaction is classic. Note that this does not mean it is a bad one or one that isn’t worth doing. It’s a classic because it is so good, easy, and should be done over and over. This is a great chemical reaction because of its safety and obvious fast results that kids can clearly see.
States of Matter
The first thing you can talk about with this reaction is the states of matter we see. We mix a solid, the baking soda, with a liquid, the vinegar. The result is bubbles, which are trapped gas. Here are some definitions to help:
- Solids: Solids hold their shape. They don’t flow or take the shape of their containers. They have strong intermolecular forces that hold the material together.
- Liquids: Liquids take the shape of the container they are in. They have intermolecular forces that hold the material together.
- Gases: Gases take the shape of their container. They do not have strong intermolecular forces, so they do not stay together as a continuous mass.

Chemical Reaction
The awesome bubbles and fizz that come from mixing baking soda and vinegar are due to the chemical reaction between the two materials.
- Chemical Reaction: A chemical reaction is a process in which a material (or more than one material) is converted to another material.
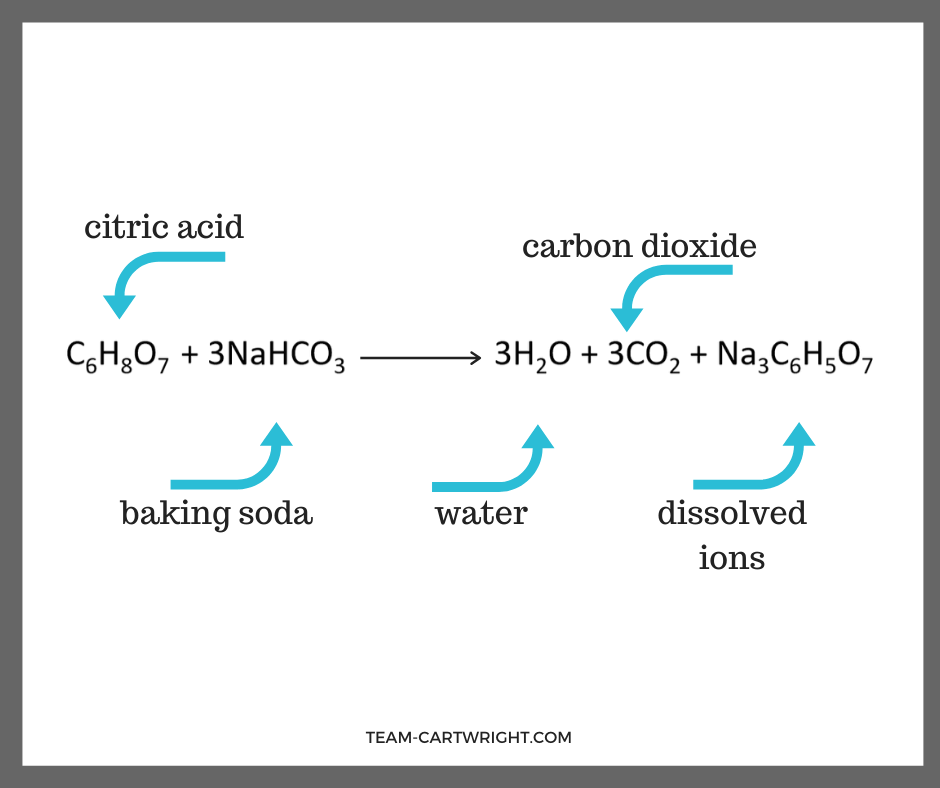
When we mix vinegar and baking soda we create carbon dioxide, water, and dissolved ions. (The dissolved ions are the atoms that don’t make the water or carbon dioxide. They remain dissolved in the water.)
Here is what that looks like as a chemical equation.
The Glitter Part
This reaction is fun on its own, but how does the glitter come up from the bottom and appear on the top?
The reason is surface tension.
- Surface Tension: Surface tension is when the attraction between the molecules on the surface of a liquid is very strong and is able to support weight.
Glitter is tiny and the surface tension of the bubbles formed is strong enough to hold onto the glitter and bring it up to the surface. This is what gives our reaction the firework quality of sparkles and flash.
Fireworks Fun Facts
Fireworks are super cool. Here are some fun facts about them your kids will love!
How Do Fireworks Work?
Fireworks are the result of small pieces of burning metal that has been sent up into the air. They start with a lifting charge to get them off the ground. A timed fuse is lit at the same time that, once in the air, ignites the bursting charge. This bursting charge lights the chemicals that actually cause the beautiful fireworks we see and they safely burst way up in the sky.
This is the really short version, obviously, the exact mechanisms are a little more complicated than that. But as fireworks are explosives they should be handled with care and only by those well trained in how to handle them safely.
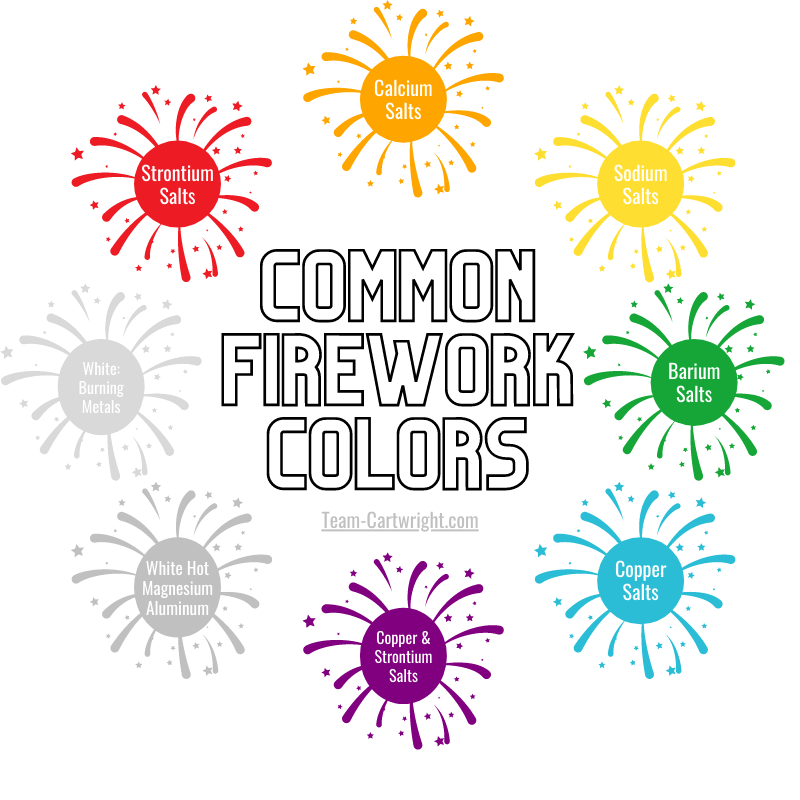
Common Firework Colors
Fireworks come in several fun colors, and those colors are the result of different salts. These sorts of salts aren’t the table salt we eat with. Salts, in chemistry, have a metal cation and a non-metal anion. (Cations have a positive charge, anions have negative, and we know positive and negative charges like to get together.)
Here are the most common firework colors:
- Red: Strontium Salts
- Orange: Calcium Salts
- Yellow: Sodium Salts
- Green: Barium Salts
- Blue: Copper Salts
- Purple: Copper and Strontium Salts
- Silver: Magnesium and Aluminum, white hot
- White: Burning Metals
Firework Fun Facts
Fireworks have been around for a very long time. Here are just a couple of fun facts about them.
- The earliest fireworks can be traced back to China in about 200 BC.
- The first fireworks using pyrotechnics were invented in China between 600-900 BC.
- The aerial shells most fireworks use today were first invented in Italy in the 1830s.
- Fireworks come in different colors depending on their chemical makeup.
- Fireworks come in different shapes depending on how their materials are packed inside them.
- The largest firework display (so far) was on February 8, 2020, in Colorado.
- China is the world’s largest exporter of fireworks.
- New Castle, PA is the firework capital of the USA.
You can find more firework fun facts by visiting the American Pyrotechnics Association.
Safe Firework Experiment Fun!
This experiment adds sparkle to any day, and your kids will love it. Try it today!

Let’s find your next fun project!
Free Printable Directions for the Firework Experiment

Baking Soda and Vinegar Firework Experiment
Fun and easy at-home firework experiment from baking soda and vinegar!
Materials
- Baking Soda
- Vinegar
- Glitter
Tools
- Shallow Dish
- Plastic Dropper
- Cookie Cutters
Instructions
- Place your cookie cutter in your shallow dish.
- Put a layer of glitter inside the cookie cutter.
- Put a layer of baking soda on top of the glitter, covering it fully.
- Have your child use the dropper to drop vinegar into the cookie cutter.
- Watch the glitter fireworks appear!
Notes
Safety
This is a pretty safe experiment. Make sure your child doesn't eat or inhale the materials used in this experiment.
Clean Up
Wipe as much of the glitter into the garbage as you can. The rest can be rinsed down the drain.








